The i-view™ Video Laryngoscope from Intersurgical: The Key Considerations

Airway Group Product Manager,
Intersurgical Ltd.
Published in Ambulance Today, Issue 3, Volume 13, Ahead of the Curve, Education and Technology Special, Autumn 2019
Video laryngoscopy represents one of the most significant advances in airway management in recent years. With the increased emphasis placed on ensuring the first attempt at intubation is the best attempt, the role of video laryngoscopy in airway management seems secure, at least for the foreseeable future1.
Video laryngoscopes utilise the latest video and camera technology to provide an optimal (indirect) view of the larynx during the process to insert an endotracheal tube in to the patient’s trachea. There are many video laryngoscopes available, but the i-viewTM from Intersurgical is the first single use adult video laryngoscope with a Macintosh type blade. i-viewTM provides the option of video laryngoscopy, wherever and whenever the clinician may need to intubate, whether in a pre-hospital setting on a patient with a difficult airway or in the emergency room on a patient with respiratory failure. Where availability of a video laryngoscope may be limited due to the cost implications of purchasing reusable devices for multiple sites, i-viewTM provides a cost-effective solution, by combining all the advantages of a fully integrated video laryngoscope in a single use, disposable product. As i-viewTM incorporates a Macintosh blade, it can be used for direct as well as video laryngoscopy, making it ideal for use in the emergency sector, where there may be a greater potential for the airway to become soiled with blood or other fluids, obscuring the view on the screen. In such circumstances, the operator can immediately switch from indirect to direct laryngoscopy.
As with all medical devices, whether single use or reusable, deciding on the most appropriate video laryngoscope to use is not straightforward, and consideration may need to be given to a number of factors. These may include evaluation of financial, environmental and infection control related issues, as well as the clinical requirements, evidence and preferences. It is important to recognise this assessment may change according to where, when and how often the device is to be used.

Financial
Whilst a single use video laryngoscope may not initially appear to be the optimal choice from a financial perspective, in circumstances where multiple units are required, but it may not be used frequently, it may prove to be the most economic option. This might include use by a Helicopter Emergency Medical Service (HEMS) or by a paramedic on an ambulance.
Infection Control
In their safety guideline booklet (2008), ‘Infection Control in Anaesthesia 2’, The Association of Anaesthetists of Great Britain and Ireland (AAGBI)2, confirmed that, in relation to standard laryngoscopes, ‘Current practices for decontamination and disinfection between patients are frequently ineffective, leaving residual contamination that has been implicated as a source of cross-infection.’ They went on to note that, ‘Blades are also regularly contaminated with blood, indicating penetration of mucous membranes, which places these items into a high-risk category.’ They concluded that the use of single use blades was ‘to be encouraged’.
Laryngoscope handles may also become contaminated. The AAGBI’s recommendation in relation to laryngoscope handles is that they should be, ‘washed/disinfected and, if suitable, sterilised by SSDs after every use.’
There is no reason to believe the same considerations and arguments that apply to standard laryngoscope blades & handles regarding infection control, would not also apply to video laryngoscopes, since all laryngoscopes, whether direct or indirect, incorporate some form of blade and handle.
In the EMS sector, where it can be particularly difficult to determine the potential cross-infection risk prior to treatment, a single use video laryngoscope offers an ideal solution.
I understand new infection prevention and control guidelines from the AAGBI are in the final draft stage, and after comments from members have been reviewed, a final version is to be presented to the Associations Board for approval.
Environmental
Environmental considerations are more complex and less easily assessed. Whilst it is appropriate for healthcare professionals, as well as anyone else with environmental concerns, to consider the implications of using single use devices in relation to product disposal, any assessment of the environmental impact of any medical device, whether single use or reusable, needs to consider a number of factors. This should include disposal of single use devices, and reprocessing or decontamination of reusable devices, in the context of a complete Life Cycle Assessment (LCA). The considerations of an LCA may vary depending on the type of product being assessed, the range and type of information and data available and the objective of the assessment. However, typically, an LCA will usually consider the following areas:
- Raw material acquisition
- Processing & manufacturing
- Distribution & transportation
- Use, reuse and maintenance
- Recycling
- Waste management
Assessing just one element of an LCA, such as waste management, may result in misleading conclusions as to the overall environmental impact of a device. A decision also needs to be taken as to what impact factors are to be assessed and how much weight is to be given to each. Is the focus primarily on climate change and water use, or is there an interest in assessing other or additional factors, such as, ecotoxicity, eutrophication, ozone depletion or urban and natural land transformation?
A number of LCA’s have been conducted for anaesthetic and airway devices. Their conclusions vary, and the complexity of any such assessment means the LCA usually needs to be considered as hospital or organisation specific; any variation in reprocessing practices, such as the volume of water used during manual washing, the electricity consumption of different types of washer/disinfection unit, or the type of packaging material used for repacking after reprocessing, will all have an effect on the overall environmental impact. Decisions also need to be taken as to what to include and exclude. For example, should energy recovery from waste incineration or the environmental impact of Personal Protective Equipment (PPE) used by healthcare workers involved in reprocessing be included?

Of course, all products have an impact on the environment, but it is important to ensure the environmental assessment is considered alongside other key factors, such as infection control considerations and the clinical benefits offered by the device.
For example, the weight given to the clinical benefit of having a single use video laryngoscope available in a life-threatening road-side emergency, perhaps when this might be the only viable VL option economically, might be quite different than the assessment made for regular routine use in the operating theatre.
In an interesting paper published in the British Journal of Anaesthesia, entitled, ‘A national survey of video laryngoscopy in the United Kingdom’, Cook & Kelly3 reported on the results of an electronic survey sent to all UK National Health Service Hospitals. With regard to availability of video laryngoscopy (VL) by clinical area, 91% of operating theatres reported availability of VL. In contrast, only 55% of Obstetric departments, 54% of Intensive Care Units and 35% of Emergency departments reported availability of VL. The authors noted that, ‘The distribution of availability is notable because the incidence of difficult or failed intubation increases in those places where video laryngoscopy is less available; in order, main theatres, obstetric, ICU, and the ED.’
It is not known why VL was less available in these areas, but it is possible that with less frequent use than in the OR, the financial implications of purchasing a reusable VL may have been a factor. If so, availability of a single use device might provide a more economically viable option due to its lower unit cost, which as discussed earlier, may be more economic when use is infrequent.
In summary, the i-viewTM video laryngoscope from Intersurgical is the first single use adult video laryngoscope with a Macintosh type blade. It provides the option of video laryngoscopy, wherever and whenever the clinician may need to intubate. This makes VL a viable option in places where the higher initial costs of purchasing a reusable device may previously have been prohibitive. With the new focus in airway management of ensuring the first attempt at intubation is the best attempt, i-viewTM may have a contribution to make to support this objective. Whilst it may not be suitable in all situations, such as when a hyper-angulated blade is required, it may be ideal in situations where use is infrequent, standard blade geometry is preferable and the nature of use makes it a more viable option economically.
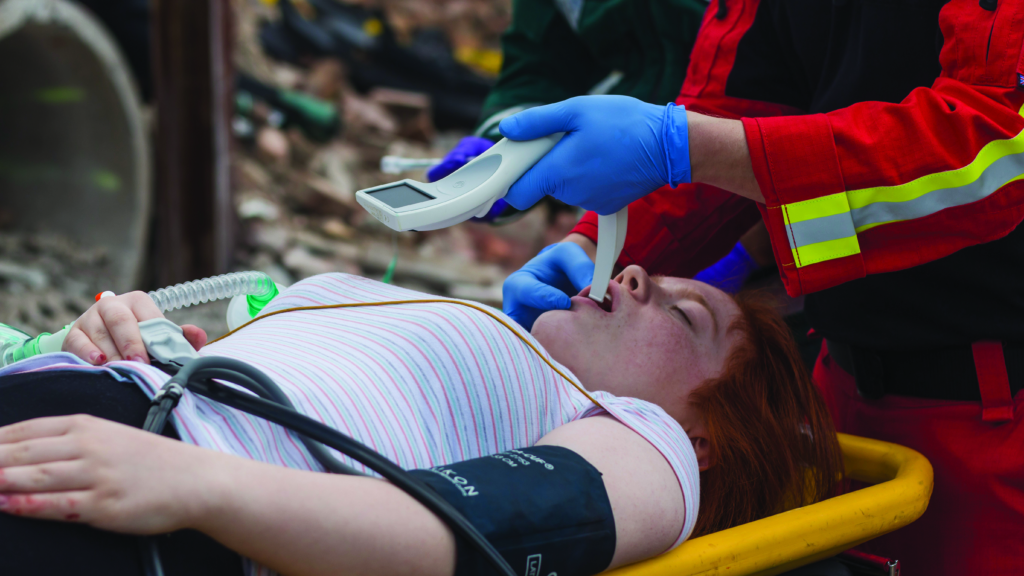
Deciding on the most appropriate video laryngoscope to purchase and use is not straightforward, and in addition to the clinical requirements and preferences, consideration may need to be given to a number of other factors, including financial, environmental and infection control related issues. It is important to recognise this assessment may change according to where, when and how often the device is to be used.
Make a direct enquiry about Intersurgical’s i-viewTM here
References:
1. Cook TM & Kelly FE. Seeing is believing: getting the best out of videolaryngoscopy. British Journal of Anaesthesia 117 (S1): i9–i13 (2016)
2. Infection Control in Anaesthesia 2. Association of Anaesthetists of Great Britain & Ireland. 2008
3. Cook TE & Kelly FE. A national survey of videolaryngoscopy in the United Kingdom. British Journal of Anaesthesia, 118 (4): 593–600 (2017)
Quality content
- Casinos Not On Gamstop
- Casinos Not On Gamstop
- Casino Sites Not On Gamstop
- Non Gamstop Casino
- UK Online Casinos Not On Gamstop
- Casino Sites Not On Gamstop UK
- Casino Sites Not On Gamstop
- Games Not On Gamstop
- Sites Not On Gamstop
- UK Online Casinos Not On Gamstop
- Casino Not On Gamstop
- Slots Not On Gamstop
- Casino Not On Gamstop
- Gambling Not On Gamstop
- Casinos Not On Gamstop
- Non Gamstop Casino
- UK Online Casinos Not On Gamstop
- Casino Sites Not On Gamstop
- Best Betting Sites
- Best UK Online Casinos
- New Horse Racing Betting Sites


Leave a Reply
Want to join the discussion?Feel free to contribute!